Building vocabulary components of an atom takes center stage, this opening passage beckons readers into a world crafted with good knowledge, ensuring a reading experience that is both absorbing and distinctly original.
The concept of an atom and its fundamental building blocks are essential to understanding the structure and behavior of matter. This guide will provide a comprehensive overview of the components of an atom, including protons, neutrons, and electrons, and their role in determining an atom’s properties.
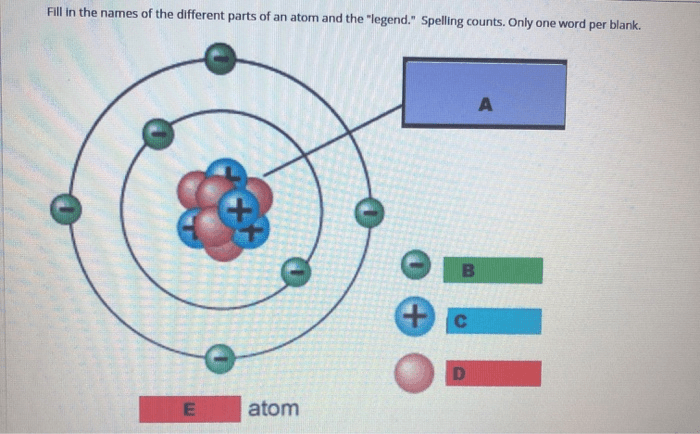
Understanding the Components of an Atom
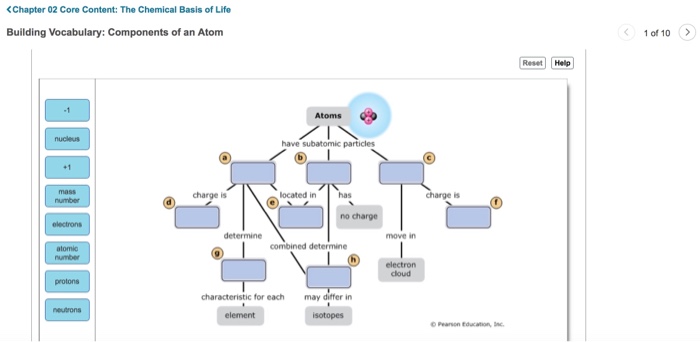
An atom is the fundamental building block of matter. It consists of three fundamental particles: protons, neutrons, and electrons.
Protons are positively charged particles located in the nucleus of an atom. Neutrons are neutral particles also located in the nucleus. Electrons are negatively charged particles that orbit the nucleus in energy levels.
Role of Protons, Neutrons, and Electrons
- Protons determine an atom’s atomic number, which identifies the element.
- Neutrons contribute to an atom’s mass but do not affect its chemical properties.
- Electrons determine an atom’s chemical reactivity and participate in chemical reactions.
Exploring the Structure of Atoms: Building Vocabulary Components Of An Atom
Atomic Structure
| Particle | Charge | Location |
|---|---|---|
| Proton | Positive | Nucleus |
| Neutron | Neutral | Nucleus |
| Electron | Negative | Orbits nucleus |
Atomic Number and Mass Number, Building vocabulary components of an atom
- Atomic number: The number of protons in an atom, determining its element.
- Mass number: The sum of protons and neutrons in an atom.
Isotopes
Atoms of the same element can have different numbers of neutrons, known as isotopes. Isotopes have the same atomic number but different mass numbers.
Building Vocabulary for Atom Components
Glossary
- Proton:A positively charged particle in the nucleus.
- Neutron:A neutral particle in the nucleus.
- Electron:A negatively charged particle that orbits the nucleus.
- Atomic number:The number of protons in an atom.
- Mass number:The sum of protons and neutrons in an atom.
- Isotope:Atoms of the same element with different numbers of neutrons.
Atomic Vocabulary in Practice
The vocabulary of atom components is essential for describing different atoms. For example, carbon has an atomic number of 6, indicating that it has 6 protons. It has 6 neutrons, giving it a mass number of 12.
Isotopes of carbon include carbon-12 (6 protons, 6 neutrons), carbon-13 (6 protons, 7 neutrons), and carbon-14 (6 protons, 8 neutrons).
Visualizing Atom Components
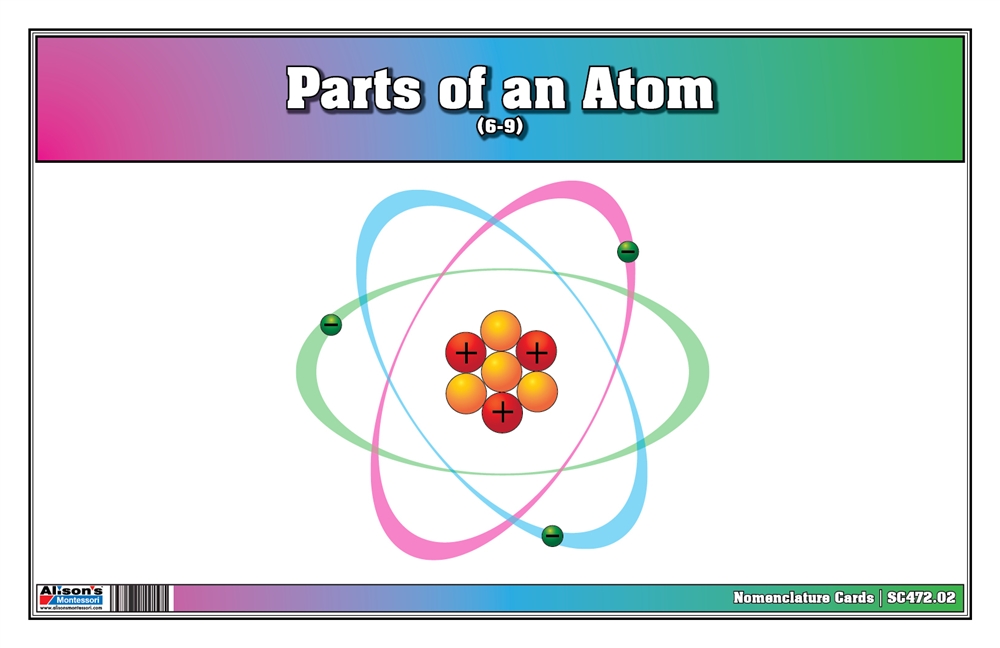
Infographic
[Infografis atau tabel yang menggambarkan komponen atom dengan jelas dan ringkas]
Building Blocks of Elements
The components of an atom determine the properties of an element. For example, the number of protons determines an element’s chemical reactivity, while the number of electrons affects its electrical properties.
Elements with the same number of protons but different numbers of neutrons have different physical properties, such as density and melting point.
Atom Components and Chemical Reactions
The components of an atom play a crucial role in chemical reactions. Changes in the number of protons, neutrons, or electrons can lead to different chemical reactions.
For example, the loss of an electron (oxidation) can change an atom’s chemical reactivity and lead to the formation of new compounds.
Popular Questions
What are the fundamental building blocks of an atom?
The fundamental building blocks of an atom are protons, neutrons, and electrons.
What is the role of protons in an atom?
Protons are positively charged particles that reside in the nucleus of an atom. They determine the atomic number of an atom and contribute to its mass.
What is the difference between isotopes?
Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons.