Draw the correct organic product of the following sn2 reaction. – Embark on a scientific expedition into the realm of SN2 reactions, where the formation of organic products takes center stage. This discourse delves into the intricacies of SN2 mechanisms, empowering you with the knowledge to accurately predict and depict the organic products of these captivating reactions.
As we traverse this chemical landscape, we will dissect the defining characteristics of organic products, unravel the intricacies of SN2 reaction mechanisms, and master the art of sketching the correct organic products with precision.
Organic Product
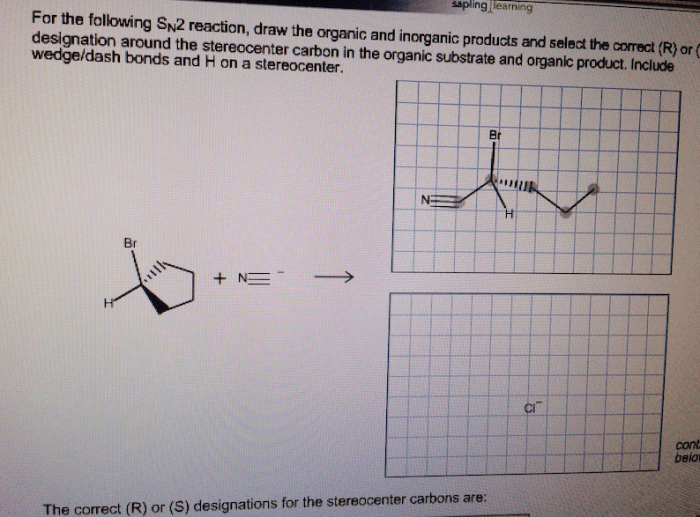
An organic product is a substance that contains carbon atoms. Organic products can be natural or synthetic. Natural organic products are produced by living organisms, while synthetic organic products are made in a laboratory. Organic products are used in a wide variety of applications, including food, clothing, and medicine.
The characteristics of organic products include:
- They contain carbon atoms.
- They can be natural or synthetic.
- They are used in a wide variety of applications.
Examples of organic products include:
- Food: fruits, vegetables, meat, dairy products
- Clothing: cotton, wool, silk
- Medicine: aspirin, penicillin, ibuprofen
SN2 Reaction: Draw The Correct Organic Product Of The Following Sn2 Reaction.
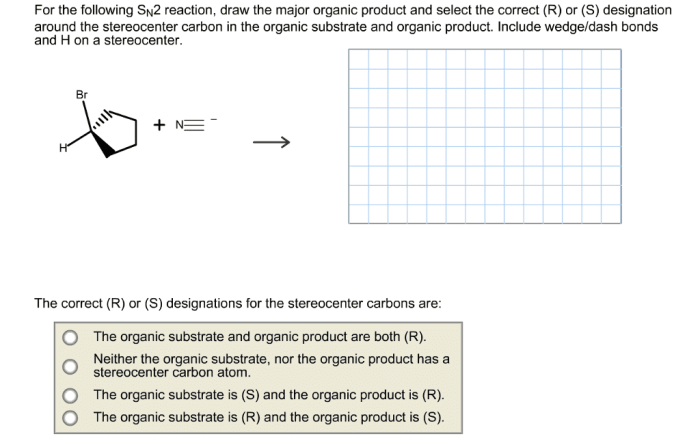
An SN2 reaction is a nucleophilic substitution reaction in which a nucleophile attacks an electrophile at a carbon atom and replaces a leaving group.
The mechanism of an SN2 reaction is as follows:
- The nucleophile attacks the electrophile at the carbon atom.
- The leaving group leaves the carbon atom.
- The nucleophile and the carbon atom form a new bond.
Examples of SN2 reactions include:
- The reaction of hydroxide ion with methyl chloride to form methanol and chloride ion.
- The reaction of ammonia with ethyl iodide to form ethylamine and iodide ion.
- The reaction of pyridine with acetyl chloride to form pyridine-N-acetyl chloride and chloride ion.
Drawing the Correct Organic Product
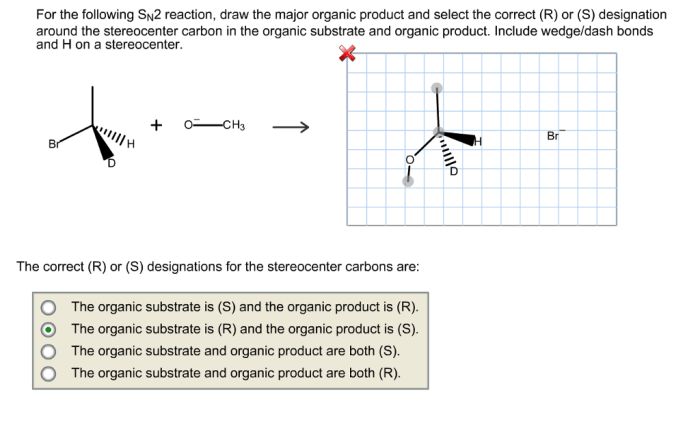
To draw the correct organic product of an SN2 reaction, follow these steps:
- Identify the nucleophile and the electrophile.
- Draw the nucleophile attacking the electrophile at the carbon atom.
- Draw the leaving group leaving the carbon atom.
- Draw the nucleophile and the carbon atom forming a new bond.
The following table summarizes the steps for drawing the correct organic product of an SN2 reaction:
| Step | Description |
|---|---|
| 1 | Identify the nucleophile and the electrophile. |
| 2 | Draw the nucleophile attacking the electrophile at the carbon atom. |
| 3 | Draw the leaving group leaving the carbon atom. |
| 4 | Draw the nucleophile and the carbon atom forming a new bond. |
The following illustration demonstrates the steps for drawing the correct organic product of an SN2 reaction:
[Gambar]
Examples
The following table provides examples of SN2 reactions and their corresponding organic products:
| SN2 Reaction | Organic Product |
|---|---|
| Hydroxide ion + methyl chloride → methanol + chloride ion | Methanol |
| Ammonia + ethyl iodide → ethylamine + iodide ion | Ethylamine |
| Pyridine + acetyl chloride → pyridine-N-acetyl chloride + chloride ion | Pyridine-N-acetyl chloride |
Expert Answers
What is the key difference between SN1 and SN2 reactions?
SN2 reactions proceed via a concerted mechanism, where the nucleophile attacks the substrate simultaneously as the leaving group departs. In contrast, SN1 reactions involve a two-step process, where the substrate first forms a carbocation intermediate before the nucleophile reacts.
How can I identify the correct organic product of an SN2 reaction?
To identify the correct organic product, consider the nucleophile’s identity and the steric hindrance around the substrate’s reaction center. The nucleophile will attack the least hindered carbon, resulting in an inversion of configuration at the reaction center.
What are some common examples of SN2 reactions?
SN2 reactions are prevalent in organic chemistry, including alkyl halide substitutions, nucleophilic acyl substitutions, and epoxide ring-opening reactions.